arsenic valence electrons|Arsenic Valence Electrons : Baguio Arsenic is the 33rd element in the periodic table and has a symbol of As and atomic number of 33. It has an atomic weight of 74.92159 and a mass number of 75. Arsenic has thirty .
Personal life Andalio was born to parents Leonardo Q. Andalio and Sandie F. Andalio, the third child of four siblings. [ Wikipedia ] Born Josefina Loisa Francisco Andalio April 21, 1999 (age 25) Filmography. My Teacher 2022. James & Pat & Dave 2020. Fantastica 2018. Hospicio 2018.
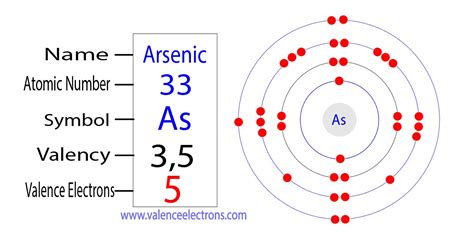
arsenic valence electrons,Mar 23, 2023 Since the last shell of an arsenic ion has eight electrons, the valence electrons of arsenic ions(As 3-) are eight. What is the valency of arsenic? The ability of .Arsenic Valence Electrons There are two ways to find the number of valence electrons in Arsenic (As). The first is to use the Periodic Table to figure out how many electrons Arsenic h. We are having the Arsenic Lewis dot diagram here for the same purpose. This dot diagram will let you study the numbers of valence electrons of an atom. The .

The atomic number of each element increases by one, reading from left to right. BlockElements are organised into blocks by the orbital type in which the outer electrons .
Arsenic is the 33rd element in the periodic table and has a symbol of As and atomic number of 33. It has an atomic weight of 74.92159 and a mass number of 75. Arsenic has thirty .
Arsenic can occupy electrons in its bonding and anti-bonding orbitals and can exhibit the properties of ligands by sharing its valence electrons. Thus, it shows the . Arsenic Number of Valence Electrons. Valence electrons are the number of electrons that are present at the outermost shell of the atom or molecules. In the case of Arsenic, there are 5 electrons in the outer .Arsenic is a chemical element of the periodic table with chemical symbol As and atomic number 33 with an atomic weight of 74.9216 u and is classed as metalloid and is part of . This table of element valences includes the maximum valence and most common valence values in chemistry. Use this for reference with a periodic table. . You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by . Arsenic-3, (+2), +3, +5: . The electronic configuration of the stable form of arsenic, As(0), can be written as shown below to illustrate how the presence of five valence electrons allows arsenic to participate in chemical bonding, with an empty p orbital for electron occupation; this can also be illustrated by the electron dot model of arsenic (Figure 1–1).Arsenic is used as the group 5 element in the III-V semiconductors gallium arsenide, indium arsenide, and aluminium arsenide. [36] The valence electron count of GaAs is the same as a pair of Si atoms, but the band .arsenic valence electronswe can calculate the number of electrons present in the valence shell by knowing the electronic configuration of Arsenic; The electron configuration for the ground-state neutral arsenic atom is given by; 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 3 or [Ar] 3 d 10 4 s 2 4 p 3. As, it has 5 electrons in its outermost valence shell (4 t h .
Quels sont les éléments de valence de l’arsenic. Les électrons de valence sont le nombre d’électrons dans la dernière orbite (coquille). La valence de l’arsenic est la somme de tous les électrons de la dernière couche suivant la configuration électronique.Arsenic is the 33rd element in the periodic table and has a symbol of As and atomic number of 33. It has an atomic weight of 74.92159 and a mass number of 75. Arsenic has thirty-three protons and forty-two neutrons in its nucleus, and thirty-three electrons in four shells. . Valence Electrons: 5 .
Use the group numbers to determine the number of valence electrons. The Group number of a non-transition metal can be used to find the number of valence electrons in an atom of that element. The ones place of the group number is the number of valence electrons in an atom of these elements. In other words: Group 1: 1 valence . Arsenic has 5 valence electrons because there are 5 electrons present in the outermost shell of the Arsenic (As) atom. Now let’s see how you can easily find the valence electrons of Arsenic atom (As). If you don’t want to read the texts, then you can also watch this video.
Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons . Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. For the main group elements (groups designated .
Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.
The electrons that determine valence – how an atom reacts chemically – are those with the highest energy.. For a main-group element, the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n. [1] Thus, the number of valence electrons that it may have depends on the electron .
Now, the electron configuration of arsenic shows that the last shell of arsenic has five electrons. Therefore, the valence electrons of arsenic are five. The last electron of arsenic enters the p-orbital. .

Comprehensive information for the element Arsenic - As is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions. . Valence Electrons: 4s 2 p 3 Electron Dot Model. Chemical Properties of Arsenic. Electrochemical Equivalent: 0.93177g/amp-hr; Again, Valency is determined from the electron configuration of the element in the excited state. The electron configuration of sulfur excited state is S*(16) = 1s 2 2s 2 2p 6 3s 2 3p x 1 3p y 1 3p z 1 3d xy 1.Here, sulfur has four unpaired electrons.
arsenic valence electrons Arsenic Valence Electrons Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd .Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.
Orbital number of the subshell. The value of ‘l’ is from 0 to (n – 1). The sub-energy levels are known as s, p, d, and f. The orbital number of the s-subshell is one, three in the p-subshell, five in the d-subshell, and seven in the f-subshell.
arsenic valence electrons|Arsenic Valence Electrons
PH0 · Valence Electrons Chart for All Elements
PH1 · How to Find the Valence Electrons for Arsenic (As)?
PH2 · How to Find the Valence Electrons for Arsenic (As)
PH3 · How many valence electrons does Arsenic have?
PH4 · How Many Valence Electrons Does Arsenic (As) Have?
PH5 · Arsenic: Chemistry, Occurrence, and Exposure
PH6 · Arsenic Valence Electrons
PH7 · Arsenic Electron Configuration (As) with Orbital Diagram
PH8 · Arsenic (As)
PH9 · Arsenic